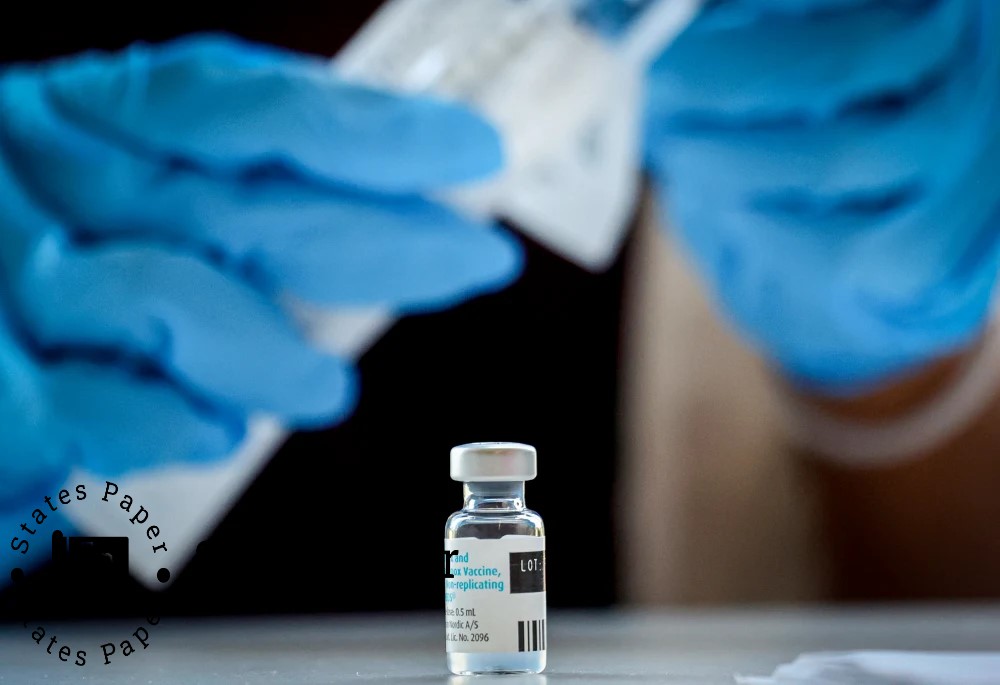
FDA approves nasal spray as first needle-free treatment for anaphylaxis

The Food and Drug Administration has granted emergency use for the ARS Pharmaceuticals nasal spray treatment for non-injectable allergic reactions, the agency said on Friday.
It is for this market that Neffy spray is being developed contrary to EpiPen and similar autoinjectors such as Kaleo’s Auvi-Q that contains epinephrine; a life-saving drug for those with anaphylaxis and other allergy risks.
Anaphylaxis is a severe allergic reaction that usually affects a lot of parts of the body and should be treated as an emergency.
Neffy is taken as a single dose of a nasal spray in one of the nostrils for patients with the age of 18 years and more and with the weight not lower than 66 pounds.
They include fear, especially if the patient is a child, and in this case, the availability of the nasal spray form of the medication may help a lot in reducing the resistance occasioned by the fear of injections by the patient, according to Kelly Stone, an associate director at the FDA’s Centre for Drug Evaluation and Research.
Neffy’s efficacy has been approved by four trials performed in 175 healthy subjects without anaphylaxis that were assessing Neffy and approved epinephrine injection formulations concerning the blood epinephrine concentrations.
In due course, it [FDA] summarily rejected the spray last year and asked for new tests to be conducted in a motion that shocked its independent experts.

 Asif Reporter
Asif Reporter