Compounding pharmacies can resume making tirzepatide as FDA reconsiders shortage
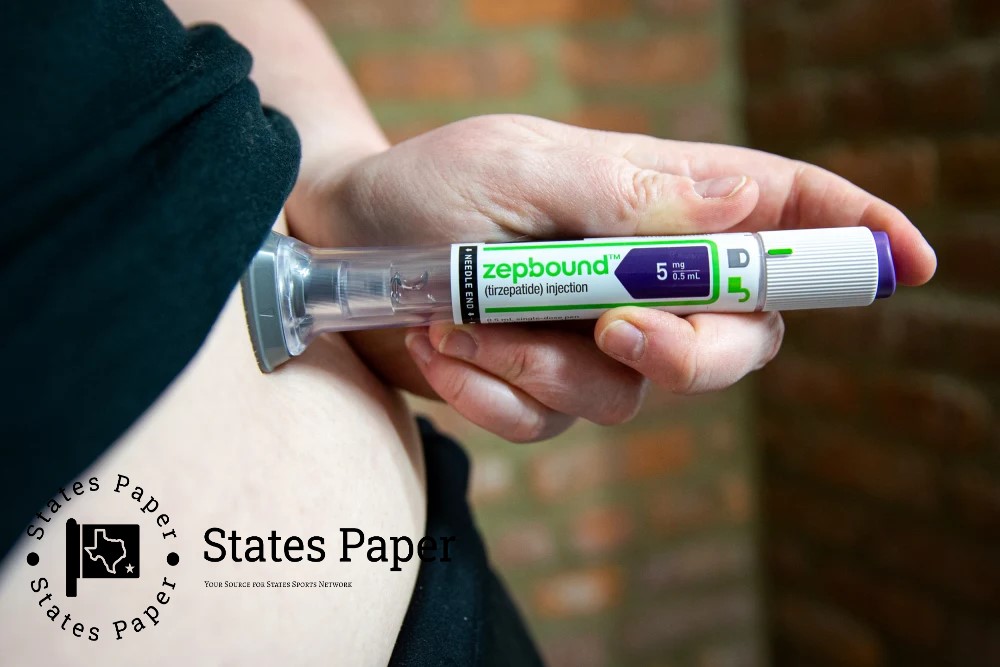
The Food and Drug Administration said in a court filing at the end of last week that it would allow pharmacists to continue making compounded versions of tirzepatide — the active ingredient in Eli Lilly’s diabetes and weight loss drugs Mounjaro and Zepbound — while it thinks through its decision to take it off its list of drugs in shortage across the country.
The decision is a coup for the compounding pharmacists and patients, who were angered by the FDA statement on October 2 that the tirzepatide shortage has been fixed.
A shortage of both the drug and semaglutide – the active ingredient in Ozempic and Wegovy – has led to unprecedented demand for compounding pharmacies to make their own version of the medicines, which than patients say are cheaper and easier to get than the branded versions.
While making versions during the FDA-declared shortages, it is legal for compounding pharmacies to make versions that are “essentially a copy” of the brand-name drugs in shortage though drugmakers have fiercely protested against such actions.
When the FDA declare on October 2, 2020 that the shortage no longer exist, compounding pharmacists could no longer fill tirzepatide prescriptions. It said at the time that pharmacies that manufacture large quantities would not be able to take new tirzepatide orders and had 60 days to fulfill existing orders.
On oct 7 the Outsourcing Facilities Association which is a compounding trade group sued the FDA stating that the drug is still in short supply and as such should be on the shortage list.
The FDA reportedly filed it on Friday in response to the group’s suit in which the latter said that the former’s action was “effectively the relief that Plaintiffs sought in their motion.” In the filing, the same agency said that, at least for the moment, it will not ‘act’ against the plaintiffs and their members who compound versions of the drugs as it undergoes the change in its decision.
Simone Williams, 50, of Spartanburg, South Carolina said she had been deeply upset when FDA removed tirzepatide from the shortage list.
Williams first administered compounded tirzepatide for weight loss last year when the Mounjaro savings card stopped being relevant. She simply cannot pay the out-of-pocket expense of one thousand, sixty dollars for the brand name of the drug she needed.
That meant Williams would have to get in there and play switcheroo and knock off semaglutide prescription waiting to be compounded due to the shortage.
Still, Williams is skeptical for, as she pointed out, the FDA’s update is “good news”.
“Until the FDA gets its act together and says there is no shortage, I am still going to be panicked and fighting for this because they can turn around and say, no there is no shortage.”
Is tirzepatide really in shortage?
Tirzepatide was among the FDA’s drug shortage list for close to two years. Originally developed as Mounjaro for Type 2 diabetes, religions were using it off label for weight loss by prescribed tirzepatide. When it was approved for such use last year –under the name Zepbound – the drug quickly became popular and thereby worsened the supply problems.
It will be recalled that Lilly has been gearing up for more tirzepatide production and has recently sighted billions to build a new factory with the major goal of producing more tirzepatide.
Michael Ganio from the American Society of Health-System Pharmacists said his group is still getting complaints from patients and caregivers stating tirzepatide is still scarce today.
Surprisingly, according to the ASHP, tirzepatide remains in shortage, although Ganio noted that that might shift when those concerned with pharmacy are informed that there is stock.
‘That’s the kind of thing that can take just a few weeks for supply to right itself,’ he said. They restock distribution centers and then distribute it to the nearest pharmacies.” The pharmacies probably all have back orders and they have customers that maybe have been getting it from the compounding pharmacy and are now trying to get it from a retail pharmacy and community pharmacy.”
Dr. Christopher McGowan, a gastroenterologist who operates a weight clinic in Cary, North Carolina, explained that he thinks the litigation is much more about the business of compounded GLP-1 than its availability, or lack thereof, on the market.
“It’s about money,” McGowan said. The compounding industry will argue that the supply chain isn’t stable enough, yet, but really there are many millions at stake. That’s the issue.”
Lilly says that tirzepatide is not in shortage.
In a statement Monday, Jared Shapiro, a Lilly spokesperson, said all doses of Mounjaro and Zepbound are available, noting that it’s dangerous for patients “not to be exposed to the risks in taking untested, unapproved knockoffs.”
“Something does not alter the truth about Mounjaro and Zepbound availability and that as per FDA, end of it, the shortage is ‘resolved’,” Shapiro said.
The FDA did not reply to a request for further input on its reversal.
An FDA spokesperson said in the statement before the Friday evening filing that the agency knows the patients’ concerns regarding its decision to call it off a shortage, the high drug prices directly affecting the patients.
“Too many Americans are priced out of the medicines they need,” a spokesperson from the committee that drafted the legislation added. “However, as a regulatory body, the FDA has no legal right to probe or regulate the prices that manufacturers, distributors and retailers fix.”
The FDA’s compounding program is designed to ensure that patients are able to continue to get ‘lawfully marketed compounded drugs’ but the agency advises that whenever possible, patients should use approved drugs.
‘Peace of mind’
On the other hand, patients and compounding pharmacists are rejoicing over the FDA’s decision that tirzepatide compounding is not prohibited.
An Outsourcing Facilities Association official who filed the case said it was glad to note that its members and the “numerous patients they treat” would benefit.
“In our view, this is a reasonable outcome given the agency’s reckless move of deleting the drug list at a time when the agency has said there are ‘supply disruptions.’” “Most important, should the FDA repeat its removal decision when a shortage still genuinely exists, it will go back to court.”
Another industry group, the Alliance for Pharmacy Compounding, called this an “an extraordinary win” for the Outsourcing Facilities Association.
In a press briefing held by APC today with reporters early this month, compounding pharmacies complained that the FDA’s initial action had an effect of ‘catching it off guard and leaving the patients flat-footed.”
Lilly had first suggested back in August that the tirzepatide issue would soon be resolved , two months before an official FDA ruling.
The APC stated in an email to its clients over the weekend that it is consulting legal advisers to establish whether the FDA intends not to take enforcement action against all compounding pharmacies, which manufacture tirzepatide, or only the plaintiffs in the case.
“What we would like to do at this juncture is to ensure that FDA undertakes enforcement discretion with regard to all pharmacies compounding copies of tirzepatide injection,” the APC wrote in the email.
Elizabeth Kenly, 59, from Graham, North Carolina said FDA approval brings her “peace of mind.”
Kenly was prescribed a compounded version of tirzepatide in March since she struggled to get Wegovy due to its availability in shortage.
The other week, the compounding pharmacists that supply Kenly contacted her to declare that tirzepatide had been removed from the shortage list and would require them to stop preparing the compounded semi-sensitives or look for another supplier. The pharmacists stated that they would use the remaining stock to dispense and aimed at providing active prescriptions for the year end.
Friday’s filing by the FDA enables Kenly not to look for any plan B.
Since she started the compounded drug, she says she has lost 30 pounds and she intends to lose another 25.
“The compounding pharmacy is, therefore, my lifeline towards continuing a treatment that has provided high value to me,” Kenly said. “While, I do know that R & D has to take profit, they also need to be partners with insurance firms and make these medications available to everyone in equal measure.”

 Asif Reporter
Asif Reporter